
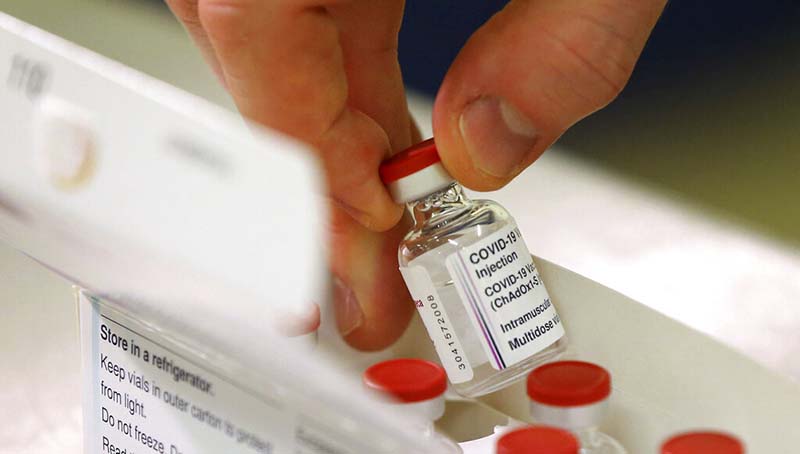
The Government of Nepal has approved the AstraZeneca vaccine manufactured by India for emergency use in the treatment of COVID-19.
Issuing a press release today, the Department of Drug Administration (DDA) officially granted permission to use the COVID-19 vaccine, ‘COVISHIELD’ manufactured by Serum Institute of India Pvt Ltd, for emergency use.
The vaccine ‘Covishield’ is also known as SARS-CoV-2 AZD 1222 or Oxford/AstraZeneca Vaccine.
In the details of the medicine, the statement describes the vaccine as the solution for injection and shows Presentation: Multi-dose Glass vial (10 dose- 5ml) and Route of Administration: Intramuscular. Other details include active and inactive ingredients of the vaccine.